The World Health Organization (WHO) has issued a call to manufacturers of mpox in vitro diagnostics (IVDs) to submit an expression of interest for Emergency Use Listing (EUL). This move is part of WHO's ongoing efforts to ensure that effective diagnostic tools are available, particularly in low-income regions where the need is most critical.
The EUL procedure allows WHO to evaluate and approve medical products, such as vaccines, tests, and treatments, for use in emergency situations. This process is vital for countries that may not have approved these products through their national regulatory systems, enabling them to procure the necessary tools to combat the virus effectively.
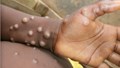
Since the outbreak of mpox, also known as monkeypox in 2022, testing has been critical in enabling early treatment, care, and the prevention of further spread. WHO has already distributed approximately 150,000 diagnostic tests worldwide, with a substantial portion directed to countries in the African Region. This support is set to continue, with an additional 30,000 tests scheduled for delivery to African nations in the coming weeks.
The Democratic Republic of the Congo (DRC) has seen a particularly sharp rise in suspected cases, with nearly 1,000 reported in a single week. In response, WHO has worked closely with partners to enhance diagnostic capacity in the country, establishing six new laboratories since May 2024. These labs, including two in South Kivu, are specifically equipped to diagnose the new viral strain, Ib, helping to decentralize testing from major cities to more affected provinces. As a result, testing rates in the DRC have quadrupled in 2024 compared to the previous year.
Mpox, caused by the monkeypox virus, can be transmitted to humans through contact with an infected person, contaminated materials, or infected animals. As the virus continues to spread, the expansion of diagnostic services is crucial.
To keep pace with the evolving situation, WHO has updated its diagnostic testing guidance to detect the new virus strain and is collaborating with countries to implement these changes. In addition, WHO has issued target product profiles to guide manufacturers in developing new diagnostic tests, reinforcing the need for timely and accurate testing solutions.
On August 14, 2024, WHO Director-General Dr. Tedros Adhanom Ghebreyesus declared the upsurge of mpox in the DRC and other African countries a Public Health Emergency of International Concern (PHEIC). This declaration underscores the urgent need for high-quality, accessible diagnostics.
Strengthened laboratory capacity, improved case investigation, contact tracing, and timely reporting are all essential components in controlling the outbreak.